Nearly 35 million adults in the United States suffer from chronic, refractory, low back pain with accompanying negative effects on function, mental health, quality of life and productivity.1 BurstDR™ spinal cord stimulation (SCS) is a clinically proven solution to treat patients with nonsurgical back pain (NSBP) and chronic pain following surgical intervention.1-5
Proven results
Distinctly superior††† treatment for NSBP
The DISTINCT study is the largest, randomized controlled clinical trial in SCS for nonsurgical back pain patients. The study evaluated the efficacy of BurstDR™ stimulation compared to conventional medical management* (CMM) in improving back pain and back pain related physical function. BurstDR™ stimulation demonstrated superiority to conventional medical management* (CMM) in pain relief, functional improvement, and pain-related emotional stress in non-surgical back pain (NSBP) patients.1-2

Average DISTINCT study NSBP patient diagnoses
NSBP can include many diagnoses; 54% of patients had 3 or more pain conditions and had failed conservative treatments for an average of 12 years.3
Robust and sustained responses to SCS
6-MONTH OUTCOMES OF THE DISTINCT STUDY1
72.6% of patients in the study and 85.2% OF THOSE IMPLANTED achieved significant back pain reduction compared to only 7.1% in the CMM arm**
Patients receiving BurstDR™ SCS thearpy REDUCED THEIR OVERALL PAIN BY AN AVERAGE OF 69.7%
270 patients enrolled; the primary endpoint was assessed in the first 200 enrolled patients
24-MONTH OUTCOMES OF THE DISTINCT STUDY6,7***
- Both SCS and crossover patients had improved outcomes on multiple patient reported outcomes (PROs) from implant through 24-month follow-up
- 96.5% of patients respond on 1 or more PROs
DISTINCT RCT paddle lead cohort
This subgroup analysis evaluated the treatment effect of using BurstDR™ stimulation capable SCS in NSBP patients implanted with paddle leads.
KEY RESULTS AT 12-MONTHS SHOWED:3
- Significant reductions in pain relief: 50% of patients reported an 80% or greater reduction in pain, with 82% of patients reporting at least a 50% pain reduction
- Substantially reduced disability: 85.7% of patients reported a ≥ 13-point meaningful improvement in disability, 76.2% experienced a 20-point or more decrease in disability scores
- Pain castastrophizing improved to reflect the average of a non-chronic pain population
- Patients reported a 93.7% satisfaction rate with their treatment
Proven efficacy in treating chronic pain following surgical intervention
In addition to treating NSBP, multiple clinical trials have demonstrated the effectiveness of SCS for patients with chronic pain following surgical intervention.4-5
SCS has shown to be more effective than reoperation as a treatment for persistent radicular pain after lumbrosacral spine surgery. For most patients, it eliminates the need for reoperation.4
The SUNBURST study (n=100) demonstrated that a majority of patients (60%) experienced a clinically significant reduction in pain†† with 12 weeks of BurstDR™ stimulation. 42% of patients in this study had chronic pain following surgical intervention.5

A cost-effective therapy delivering robust outcomes with full body MR conditionality
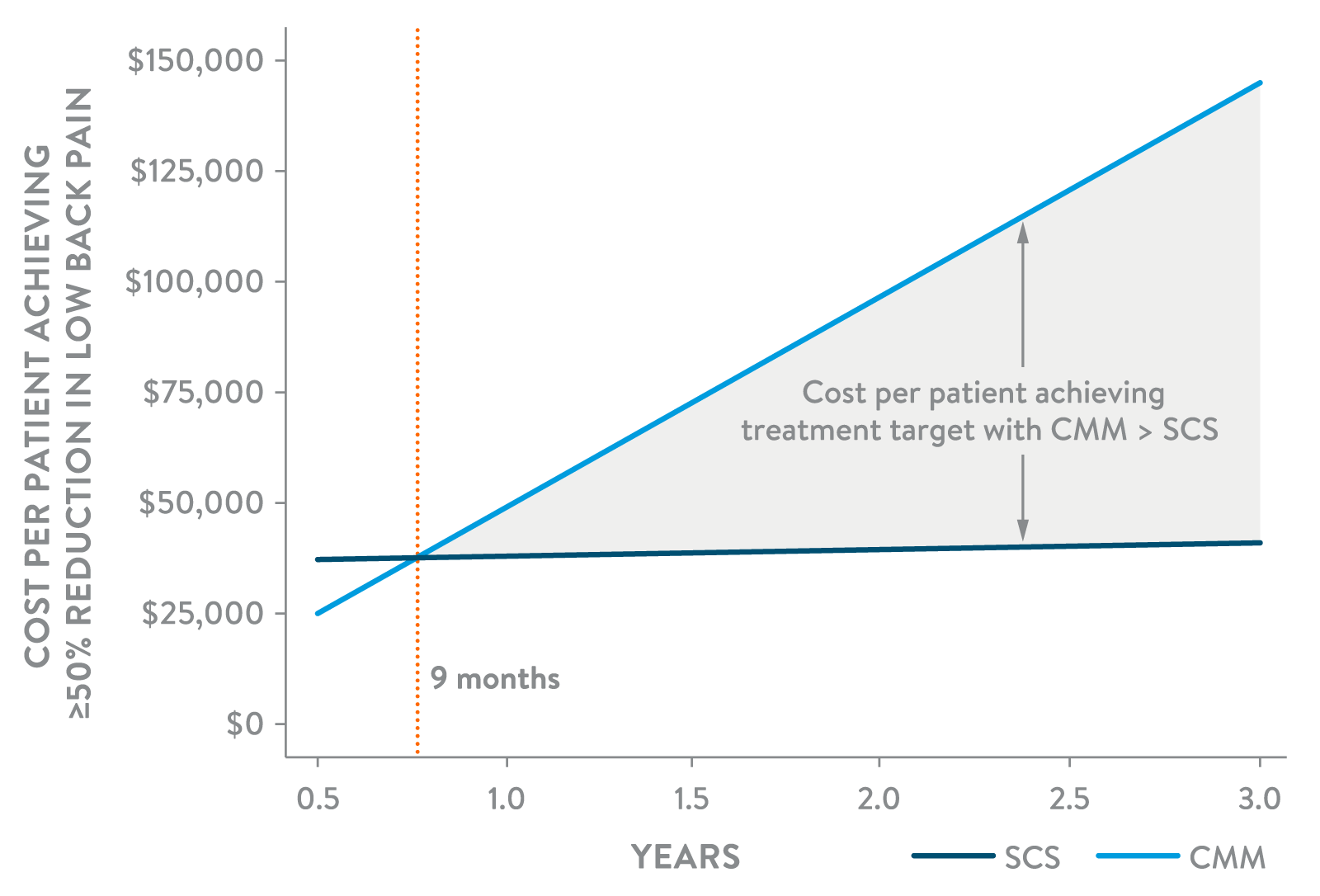
A COST-EFFECTIVE SOLUTION
SCS therapy has demonstrated improved cost-effectiveness compared to CMM and reoperation for patients with NSBP and chronic pain following surgical intervention.8-10
Among NSBP patients achieving 50% or more pain reduction, SCS demonstrated to be more cost-effective beginning at 9 months and was 3.5x LESS EXPENSIVE than CMM at 3 years.8

Full body MR conditionality†
Patients implanted with our SCS systems can safely undergo full body MRI scans.† See our MRI support page to learn more about the scan details for our MR Conditional SCS products.
Built on a proprietary waveform
WHY CHOOSE BURSTDR™ STIMULATION AND FLEXBURST360™ THERAPY?
Explore the body of evidence supporting BurstDR™ stimulation and how it provides relief from physical pain and beyond,5,11-13 treats patients effectively and delivers durable relief with a low-energy therapy,14,30 and has consistent, positive results.5,11,15-30
Choose a proven therapy. Earlier.
Currently patients with chronic refractory back pain who experience a prolonged delay of neuromodulation treatment beyond two years experience increased costs without improved outcomes.1 Consider SCS as an effective option for patients with NSBP and chronic pain following surgical intervention.

BurstDR™ Stimulation, patented technology exclusively from Abbott, is also referenced to as Burst stimulation in clinical literature.
*Subjects randomized to the CMM arm received supervised medical care, including physical modalities, medication optimization, and interventional therapies depending on the diagnosis and as decided by the investigator. Medication optimization could include using nonsteroidal antiinflammatories, anticonvulsants, muscle relaxants, opioids, and other analgesics as appropriate. Supervised noninterventional therapy could include physical therapy (including back school), chiropractic care, cognitive behavioral therapy, massage, and acupuncture. Interventional therapies, such as injections and radiofrequency therapy, also were allowed.
**≥50% improvement on NRS score from baseline.
***Our labeling includes up to 6 months of SCS safety and effectiveness data for NSBP. Data in this brochure includes follow up data to 24 months, supported by an internal clinical study report and an 2025 ASPN podium presentation.
† Within approved parameters. Refer to the Instructions for Use for full details on the MR Conditional scan parameters.
†† Clinically significant reductions were defined in the study protocol as a decrease in the overall daily VAS score from baseline by at least 30%.
††† BurstDR™ superiority compared to CMM as seen in the DISTINCT study.
- Deer T., Gilligan C., Falowski S., et al. 2023. Treatment of Refractory Low Back Pain Using Passive Recharge Burst in Patients Without Options for Corrective Surgery: Findings and Results From the DISTINCT Study, a Prospective Randomized Multicenter Controlled Trial. Neuromodulation 2023; 26: 1387–1399.
- Deer, T., Heros, R., Tavel, E., et al. (2024). Comparing conventional medical management to spinal cord stimulation for the treatment of low back pain in a cohort of distinct RCT patients. Journal of Pain Research, Volume 17, 2741–2752. https://doi.org/10.2147/jpr.s472481.
- Falowski S, Dorsi MJ, Heros R, et al. Paddle leads for the treatment of nonsurgical back pain- The DISTINCT study. Pain Pract. 2025;25(5):e70033. doi:10.1111/papr.70033.
- North RB, Kidd DH, Farrokhi F, et al. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: a randomized, controlled trial. Neurosurgery. 2005;56(1):98-106; discussion 106-7. doi: 10.1227/01.neu.0000144839.65524.e0. PMID: 15617591.
- Deer, Timothy, Konstantin V. Slavin, Kasra Amirdelfan, et al. “Success using neuromodulation with BURST (SUNBURST) study:results from a prospective, randomized controlled trial using a novel burst waveform.” Neuromodulation: Technology at the Neural Interface 21, no. 1 (2018): 56-66
- Deer et al., The DISTINCT Study: 24-Month SCS Data for Nonsurgical Low Back Pain. ASPN. Miami, FL. 2025
- Abbott. DISTINCT Clinical Study Final Report (CL1024970). 2024
- Deer, T., Heros, R., Scarfo, K., et al. (2025). A cost effectiveness analysis of spinal cord stimulation versus conventional medical management for the treatment of low back pain using data from distinct RCT and medical claims from a U.S. commercial payer database. Journal of Pain Research, Volume 18, 2823–2838. https://doi.org/10.2147/jpr.s486759
- North, R., Kidd, D., Shipley, J., et al. (2007). Spinal cord stimulation versus reoperation for failed back surgery syndrome: A cost effectiveness and cost utility analysis based on a randomized, controlled trial. Neurosurgery. https://pubmed.ncbi.nlm.nih.gov/17762749/.
- McClure JJ, Desai BD, Ampie L, You W, Smith JS, Buchholz AL. A Systematic Review of the Cost-Utility of Spinal Cord Stimulation for Persistent Low Back Pain in Patients With Failed Back Surgery Syndrome. Global Spine J. 2021;11(1_suppl):66S-72S. doi:10.1177/2192568220970163
- Leong SL, De Ridder D, Deer T, Vanneste S. Potential Therapeutic Effect of Low Amplitude Burst Spinal Cord Stimulation on Pain. Neuromodulation. 2021;24(3):574-580.
- Falowski SM, Moore GA, Cornidez EG, et al. Improved Psychosocial and Functional Outcomes and Reduced Opioid Usage Following Burst Spinal Cord Stimulation. Neuromodulation. Published online June 2021. doi:10.1111/ner.13226
- Falowski SM, Nanivadekar AC. Prospective Six-Month Analysis of Multiarea Burst Spinal Cord Stimulation: Correlating Intraoperative Neuromonitoring With Postoperative Programming and Clinical Outcomes. Neuromodulation. 2024 Mar 22:S1094-7159(24)00056-4. doi: 10.1016/j.
- Deer TR, Patterson DG, Baksh J, et al. Novel intermittent dosing burst paradigm in spinal cord stimulation. Neuromodulation. 2021;24(3):566-573. doi:10.1111/ner.13143
- Karri J, Orhurhu V, Wahezi S, Tang T, Deer T, Abd-Elsayed A. Comparison of spinal cord stimulation waveforms for treating chronic low back pain: Systematic review and meta-analysis. Pain Physician. 2020;23(5):451-460. doi:10.36076/ppj.
- De Ridder D, Vanneste S, PlazierM, van der Loo E, MenovskyT. Burst spinal cord stimulation: toward paresthesia-free pain suppression. Neurosurgery. 2010;66(5):986-990.
- Bara B, Schu S, Vesper J. First results of Burst high frequency stimulation in failed FBSS stimulation patients. One year follow up. Neuromodulation. 2013;16(5):e136.
- Espinet A, Courtney P, Mitchell B, et al. Burst spinal cord stimulation provides superior overall pain relief compared to tonic stimulation. Pain Pract. 2014;14(s1):114.
- De Vos CC, Bom MJ, Vanneste S, Lenders MW, de Ridder D. Burst spinal cord stimulation evaluated in patients with failed back surgery syndrome and painful diabetic neuropathy. Neuromodulation. 2014;17(2):152-159.
- Kriek N, Groeneweg JG, Stronks DL, de Ridder D, Huygen FJ. Preferred frequencies and waveforms for spinal cord stimulation in patients with complex regional pain syndrome: A multicentre, double-blind, randomized andplacebo-controlled crossover trial. Eur J Pain. 2017;21(3):507-519. doi:10.1002/ejp.944
- De Ridder D, Lenders MW, De Vos CC, et al. A 2-center comparative study on tonic versus burst spinal cord stimulation: amount of responders and amount of pain suppression. Clin J Pain. 2015;31(5):433-437. doi:10.1097/AJP.0000000000000129
- Kinfe TM, Muhammad S, Link C, Roeske S, Chaudhry SR, Yearwood TL. Burst spinal cord stimulation increases peripheral antineuroinflammatory interleukin 10 levels in failed back surgery syndrome patients with predominant back pain. Neuromodulation. 2017;20(4):322-330. doi:10.1111/ner.12586
- Wahlstedt A, Leljevahl E, Venkatesan L, Agnesi F. Cervical burst spinal cord stimulation for upper limb chronic pain: A retrospective case series. Poster presented at 16th Annual Pain Medicine Meeting; 2017; Lake Buena Vista, FL.
- Muhammad S, Roeske S, Chaudhry SR, Kinfe TM. Burst or high-frequency (10 kHz) spinal cord stimulation in Failed Back Surgery Syndrome patients with predominant back pain: one year comparative data. Neuromodulation. 2017;20(7):661-667. doi:10.1111/ner.12611
- Kretzschmar M, Vesper J, Van Havenbergh T, et al. Improved pain and psychosocial function with Burst SCS: 1 year outcomes of a prospective study. Neuromodulation. 2017;20(7):e450.
- Bocci T, De Carolis G, Paroli M, et al. Neurophysiological comparison among tonic, high frequency, and burst spinal cord stimulation: novel insights into spinal and brain mechanisms of action. Neuromodulation. 2018;21:480–488.
- Grider JS, Harned M. Cervical spinal cord stimulation using monophasic burst waveform for axial neck and upper extremity radicular pain: a preliminary observational study. Neuromodulation. 2020;23(5):680-686. doi:10.1111/ner.13041
- Pope JE, Schu S, Sayed D, et al. Anatomic lead placement without paresthesia mapping provides effective and predictable therapy during the trial evaluation period: results from the prospective, multicenter, randomized, DELIVERY Study. Neuromodulation. 2020;23(1):109-117. doi:10.1111/ner.13019
- Deer TR, Patterson DG, Baksh J, et al. Novel intermittent dosing burst paradigm in spinal cord stimulation. Neuromodulation. 2021;24(3):566-573. doi:10.1111/ner.13143
- Deer T, Wilson D, Schultz D, et al. Ultra-low energy cycled burst spinal cord stimulation yields robust outcomes in pain, function, and affective domains: A subanalysis from two prospective, multicenter, international clinical trials. Neuromodulation. 2022;25(1):137-144. doi:10.1111/ner.13507
Important Safety Information
Spinal Column Stimulation (SCS) Systems
Intended Use
This neurostimulation system is designed to deliver low-intensity electrical impulses to nerve structures. The system is intended to be used with leads and associated extensions that are compatible with the system.
Indications For Use
Abbott Medical spinal cord stimulation (SCS) systems are indicated as an aid in the management of chronic, intractable pain of the trunk and/or limbs, including unilateral or bilateral pain associated with the following: failed back surgery syndrome, nonsurgical back pain (without prior surgery and not a candidate for back surgery), and diabetic peripheral neuropathy of the lower extremities.
Contraindications
This system is contraindicated for patients who are unable to operate the system or who have failed to receive effective pain relief during trial stimulation. The trial neurostimulation (EPG) should not be used on patients who are poor surgical risks, such as those with multiple illnesses or active general infections.
MRI Safety Information
Some models of this system are Magnetic Resonance (MR) Conditional, and patients with these devices may be scanned safely with magnetic resonance imaging (MRI) when the conditions for safe scanning are met. For more information about MR Conditional neurostimulation components and systems, including equipment settings, scanning procedures, and a complete listing of conditionally approved components, refer to the MRI procedures clinician's manual for neurostimulation systems (available online at medical.abbott/manuals). For more information about MR Conditional products, visit the Abbott Medical product information page at http://neuromodulation.abbott/MRI-ready.
Warnings
Poor surgical risks. Neurostimulation should not be used on patients who are poor surgical candidates. Neurostimulation should not be used for patients with comorbidities that could prevent successful implant or effective therapy.
Magnetic resonance imaging (MRI). Some patients may be implanted with the components that make up a Magnetic Resonance (MR) Conditional system, which allows them to receive an MRI scan if all the requirements for the implanted components and for scanning are met. A physician can help determine if a patient is eligible to receive an MRI scan by following the requirements provided by Abbott Medical. Physicians should also discuss any risks of MRI with patients. Patients without an MR Conditional neurostimulation system should not be subjected to MRI because the electromagnetic field generated by an MRI may forcefully dislodge implanted components, damage the device electronics, and induce voltage through the lead that could jolt or shock the patient.
Diathermy therapy. Do not use short‑wave diathermy, microwave diathermy, or therapeutic ultrasound diathermy (all now referred to as diathermy) on patients implanted with a neurostimulation system. Energy from diathermy can be transferred through the implanted system and cause tissue damage at the location of the implanted electrodes, resulting in severe injury or death. Diathermy is further prohibited because it may also damage the neurostimulation system components. This damage could result in loss of therapy, requiring additional surgery for system implantation and replacement. Injury or damage can occur during diathermy treatment whether the neurostimulation system is turned on or off. All patients are advised to inform their healthcare professional that they should not be exposed to diathermy treatment
Implanted cardiac systems. Physicians need to be aware of the risk and possible interaction between a neurostimulation system and an implanted cardiac system, such as a pacemaker or defibrillator. Electrical pulses from a neurostimulation system may interact with the sensing operation of an implanted cardiac system, causing the cardiac system to respond inappropriately. To minimize or prevent the implanted cardiac system from sensing the output of the neurostimulation system, (1) maximize the distance between the implanted systems (minimum separation distance of approximately 8 cm (3 in.) between lead ends is recommended); (2) verify that the neurostimulation system is not interfering with the function of the implanted cardiac system; and (3) consider bipolar programming of both devices and use neurostimulation system settings that do not interfere with the function of the implantable cardiac system.
Electrosurgery. To avoid harming the patient or damaging the neurostimulation system, do not use monopolar electrosurgery devices on patients with implanted neurostimulation systems. If use of electrocautery is necessary, place the neurostimulator in Surgery mode using the clinician programmer app or the patient controller app before using an electrosurgery device.
During the implant procedure, if an electrosurgery device must be used, take the following actions:
- Use bipolar electrosurgery only.
- Place the neurostimulator in Surgery mode before using an electrosurgery device.
- Set the electrosurgery device to the lowest possible energy setting. Output power below 80 W is recommended for all activations.
- Complete any electrosurgery before connecting the leads or extensions to the neurostimulator.
- Keep the current paths from the electrosurgery device as far from the neurostimulation system as possible.
- Exit Surgery mode during intraoperative testing and after the procedure is completed.
NOTE: During intraoperative testing, Surgery mode must be turned off for the neurostimulation system to function correctly
- Confirm that the neurostimulation system is functioning correctly during the implant procedure, before closing the neurostimulator pocket, and after the implant procedure.
- After any surgery, check the neurostimulation system for the following:
- Check the neurostimulator to ensure Surgery mode has been turned off, even if Surgery mode was not turned on at the beginning or during the procedure.
- Confirm the neurostimulation system is functioning
Electrosurgery devices. Electrosurgery devices should not be used in close proximity to an implanted neurostimulation device. Contact between an active electrode and an implanted lead or extension can cause direct stimulation of the tissue at the electrode site and cause severe injury to the patient. If use of electrocautery is necessary, first turn off the neurostimulation system.
Other active implanted devices. The neurostimulation system may interfere with the normal operation of another active implanted device, such as a pacemaker, defibrillator, or another type of neurostimulator. Conversely, the other active implanted device may interfere with the operation of the neurostimulation system.
Operation of machines, equipment, and vehicles. Patients using therapy that generates paresthesia should turn off stimulation before operating motorized vehicles, such as automobiles, or potentially dangerous machinery and equipment because sudden stimulation changes may distract them from properly operating it. However, current data shows that most patients using BurstDR™ stimulation therapy do not experience paresthesia. For patients who do not feel paresthesia, sudden stimulation changes are less likely to occur and distract them while operating motorized vehicles, machinery, or equipment.
Explosive and flammable gases. Do not use a clinician programmer or patient controller around explosive or flammable gas fumes or vapors. This includes oxygen-enriched environments such as hyperbaric chambers. Operating the device near gas fumes or vapors could cause them to catch fire. If gas fumes or vapors catch fire, it could cause severe burns, injury, or death.
Keep dry to avoid damage. Clinician programmers, patient controllers, and chargers are not waterproof. Keep them dry to avoid damage. Advise patients to not use their devices when engaging in activities that might cause them to get wet, such as swimming or bathing.
Pediatric use. Safety and effectiveness of neurostimulation for pediatric use have not been established.
Pregnancy and nursing. Safety and effectiveness of neurostimulation for use during pregnancy and nursing have not been established.
Use in patients with diabetes. Surgical complications and adverse effects may be more frequent and severe inpatients with diabetes. The following additional considerations should be made for patients with diabetes:
- A pre-operative risk assessment should be performed for patients with diabetes who are at high risk for ischemic heart disease, those with autonomic neuropathy or renal failure, and patients with a Hemoglobin A1C (HbA1c) ≥8% (64 mmol/mol).
- Monitor the patient’s blood glucose levels in the perioperative period and instruct the patient to continue to monitor glucose levels as they may fluctuate as a response to surgery or to complications. Implanting physicians or anesthesiologists should consult practice guidelines for the intraoperative management of patients with diabetes.
- Closely monitor patients for signs of infection, delayed wound healing, or cerebrospinal fluid (CSF) leakage as the severity of these complications may be greater in patients with diabetes.
Interference with other devices. Some of this system’s electronic equipment, such as the programmer and controller, can radiate radiofrequency (RF) energy that may interfere with other electronic devices, including other active implanted devices. Avoid placing equipment components directly over other electronic devices. To correct the effect of interference with other devices, turn off the equipment or increase the distance between the equipment and the device being affected.
System components. The use of components not approved for use by Abbott Medical may result in damage to the system and increased risk to the patient
Device modification. The equipment is not serviceable by the customer. To prevent injury or damage to the system, do not modify the equipment. If needed, return the equipment to Abbott Medical for service.
Software modification. Do not modify the generator software in any way. Only apply software updates that are published directly by Abbott Medical.
Application modification. To prevent unintended stimulation, do not modify the operating system application in any way
MRI Mode. An inability to disable MRI Mode will occur if the patient controller is no longer paired to the IPG and there is no previously paired clinician programmer available or if the clinician programmer lost its pairing to the IPG. The inability to disable MRI Mode would require device replacement surgery to restore therapy for generators with the following software versions: 1.1.0.1, 1.1.1.1, 1.1.2.1.
Strangulation. The cords in this system pose a strangulation risk. To avoid strangulation, be careful when using cords and keep cords out of the reach of children.
Stimulation modes. The BurstDR™ stimulation mode has not been evaluated for effectiveness in the diabetic peripheral neuropathy (DPN) population.
Theft detectors and metal screening devices. Certain types of antitheft devices, such as those used at entrances or exits of department stores, libraries, and other public establishments, and airport security screening devices may affect stimulation. Patients who are implanted with nonadjacent multiple leads and patients who are sensitive to low stimulation thresholds may experience a momentary increase in their perceived stimulation, which has been described by some patients as uncomfortable or jolting. Patients should use caution when approaching such a device and should request assistance to bypass the device. If they must proceed through the device, patients should turn off the IPG and proceed with caution, being sure to move through the detector quickly.
Cardioverter defibrillators. Neurostimulation systems may adversely affect the programming of implanted cardioverter defibrillators.
Electromagnetic interference (EMI). Some equipment in home, work, medical, and public environments can generate EMI that is strong enough to interfere with the operation of a neurostimulation system or damage system components. Patients should avoid getting too close to these types of EMI sources, which include the following examples: commercial electrical equipment (such as arc welders and induction furnaces),communication equipment (such as microwave transmitters and high‑power amateur transmitters),high‑voltage power lines, radiofrequency identification (RFID) devices, and some medical procedures (such as therapeutic radiation and electromagnetic lithotripsy).
Additional Warnings Only Applicable to the Trial System
Power supply. Use only the CR2450 batteries supplied with the device. CR2450 batteries have a nominal voltage of 3V and nominal capacity of at least 600 mAh.
Routine medical procedures. For patients using a trial system, avoid elective medical procedures.
Additional Warnings Only Applicable to the Permanent System
Case damage. Do not handle the generator if the case is pierced or ruptured because severe burns could result from exposure to battery chemicals.
Generator disposal. Return all explanted generators to Abbott Medical for safe disposal. Generators contain batteries as well as other potentially hazardous materials. Do not crush, puncture, or burn the generator because explosion or fire may result.
Product materials. Neurostimulation systems have materials that come in contact or may come in contact with tissue. A physician should determine whether or not a patient may have an allergic reaction to these materials before the system is implanted
Additional Warnings Only Applicable to the Paddle Leads
Preoperative imaging. Preoperatively review imaging of the spine (for example, MRI or computerized tomography [CT] with or without myelography) in the region where the paddle is intended to be implanted to ensure adequate space for the lead.
Compressive lesions. Systematically assess the presence of any compressive lesions in the region where the paddle is intended to be implanted.
Precautions
General Precautions
Clinician training. Implanting physicians should be familiar with neurostimulation therapy and experienced in the diagnosis and treatment of chronic pain syndromes and have undergone surgical and device implantation training.
Patient selection. It is extremely important to select patients appropriately for neurostimulation. Thorough psychiatric screening should be performed. Patients should not be dependent on drugs and should be able to operate the neurostimulation system.
Infection. Follow proper infection control procedures. Patients should avoid charging their generator over an incision that has not completely healed. Infections related to system implantation might require that the device be explanted.
Implantation of two systems. If two systems are implanted, ensure that at least 20 cm (8 in.) separates the implanted generators to minimize unintended interaction with other system components
Implantation of multiple leads. If multiple leads are implanted, leads and extensions should be routed in close proximity. Nonadjacent leads can possibly create a conduit for stray electromagnetic energy that could cause the patient unwanted stimulation.
Stimulation parameters. Patients should be cautioned that stimulation parameters must be determined under the supervision of a physician and that they should not adjust stimulation parameters within prescribed programs except under direct orders from their physician.
High stimulation outputs. Stimulation at high outputs may cause unpleasant sensations or motor disturbances or render the patient incapable of controlling the generator. If unpleasant sensations occur, turn off stimulation immediately.
Consumer goods and electronic devices. Magnetic interference with consumer goods or electronic devices that contain magnets, such as mobile phones and smart watches, may unintentionally cause the neurostimulation system to turn on or turn off or affect communication between the device and generator; however, it will not change the prescribed programmed parameters. Patients should be advised to keep their mobile phones and smart watches at least 15 cm (6 in.) away from the generator and avoid placing any smart device in a pocket near the generator. If a patient is concerned about a smart device interacting with their neurostimulation system, consider disabling magnet mode. For more information about setting the magnet mode, refer to the clinician programmer manual or contact Technical Support.
Lead movement. Patients should be instructed to avoid bending, twisting, stretching, and lifting objects over 2 kg (5 lb) for six to eight weeks after implantation of a neurostimulation system. Extension of the upper torso or neck may cause lead movement and alter the stimulation field (especially with leads in the cervical area),resulting in overstimulation or ineffective stimulation.
Patient training. Instruct patients to use their neurostimulation system only after an authorized clinician has programmed the device and has trained the patient how to control stimulation and safely use the system
Hospital and Medical Environments
Therapeutic radiation. Therapeutic radiation may damage the electronic circuitry of an implanted neurostimulation system, although no testing has been done and no definite information on radiation effects is available. Sources of therapeutic radiation include therapeutic X‑rays, cobalt machines, and linear accelerators. If radiation therapy is required, the area over the implanted generator should be shielded with lead. Damage to the system may not be immediately detectable.
High output ultrasonics and lithotripsy. The use of high-output devices, such as an electrohydraulic lithotriptor, may damage the electronic circuitry of an implanted generator. If lithotripsy must be used, do not focus the energy near the generator. ‑output ultrasonics and lithotripsy
Ultrasonic scanning equipment. The use of ultrasonic scanning equipment may cause mechanical damage to an implanted neurostimulation system if used directly over the implanted device. External defibrillators. Safety for use of external defibrillator discharges on a patient receiving neurostimulation has not been established.
External defibrillation can cause induced currents in the lead extension portion of the neurostimulation system. After defibrillation, confirm the neurostimulation system is still working.‑extension portion of the neurostimulation system. After
Handling and Implantation
Handle the device with care. The clinician programmer and patient controller are sensitive electronic devices that can be damaged by rough handling, such as dropping it on the ground, or exposure to extreme hot or cold temperatures. For more information, refer to the user guide for the provided Apple‡ iOS‡ device available at support.apple.com/guide.
Single use, sterile device. The implanted components of this neurostimulation system are intended for a single use only. Sterile components in this kit have been sterilized using ethylene oxide (EtO) gas before shipment and are supplied in sterile packaging to permit direct introduction into the sterile field. Do not resterilize or reimplant an explanted system for any reason.‑use, sterile device
Storage environment. Store components and their packaging where they will not come in contact with liquids of any kind.
Expiration date. An expiration date (or “use‑by” date) is printed on the packaging. Do not use the system if the use‑by date has expired.
Care and handling of components. Use extreme care when handling system components prior to implantation. Excessive heat, excessive traction, excessive bending, excessive twisting, or the use of sharp instruments may damage and cause failure of the components.
Package or component damage. Do not implant a device if the sterile package, the device, or any device components show signs of damage, tampering, or if the sterile seal is ruptured, or contamination is suspected for any reason. Return any suspect components to Abbott Medical for evaluation.
Exposure to body fluids or saline. Exposure of the metal contacts, such as those on the connection end of a lead or extension, to body fluids or saline prior to connection can lead to corrosion. If such exposure occurs, clean the affected parts with sterile, deionized water or sterile water for irrigation, and dry them completely prior to lead connection and implantation.
System testing. To ensure correct operation, always test the system during the implant procedure, before closing the neurostimulator pocket, and before the patient leaves the surgery suite.
Component disposal. Dispose of the EPG header and pouch with other medical waste. Return the EPG and all explanted lead components to Abbott Medical for safe disposal when necessary
Home and Occupational Environments
Security, antitheft, and radiofrequency identification (RFID) devices. Some antitheft devices, such as those used at entrances or exits of department stores, libraries, and other public places, and airport security screening devices may affect stimulation. Additionally, RFID devices, which are often used to read identification badges, as well as some tag deactivation devices, such as those used at payment counters at stores and loan desks at libraries, may also affect stimulation. Patients who are implanted with nonadjacent multiple leads and patients who are sensitive to low stimulation thresholds may experience a momentary increase in their perceived stimulation, which some patients have described as uncomfortable or jolting. Patients should cautiously approach such devices and should request help to bypass them. If they must go through a gate or doorway containing this type of device, patients should turn off their generator and proceed with caution, being sure to move through the device quickly.
Scuba diving or hyperbaric chambers. Before diving or using a hyperbaric chamber, patients should contact their physician to discuss the effects of high pressure on their implanted system. Implanted systems with non‑Abbott Medical leads have not been evaluated for safety while scuba diving or in hyperbaric chambers. Patients with implanted Abbott Medical leads should avoid scuba diving in more than 30 m (100 ft) of water or entering hyperbaric chambers above 4.0 atmospheres absolute (ATA) for any length of time, as this may damage the neurostimulation system. For less than 30 m (100 ft) of water or pressures below 4.0 ATA, durations of less than 60 minutes are recommended.
Wireless use restrictions. In some environments, the use of wireless functions (for example, Bluetooth® wireless technology) may be restricted. Such restrictions may apply aboard airplanes, near explosives, or in hazardous locations. If you are unsure of the policy that applies to the use of this device, please ask for authorization to use it before turning it on.
Additional Precautions Only Applicable to the Rechargeable System
Implant heating. While charging the generator, patients may perceive an increase in temperature at the generator site. In patients who have areas of increased sensitivity to heat, consider placing the implant where the patient has normal sensation.
Recharge-by date. A recharge‑by date is printed on the packaging. If this date has been reached or has been exceeded before the date of implantation, the generator should be charged prior to implantation.
Additional Precautions Only Applicable to the Clinician Programmer System
Nonsterile device. The clinician programmer is a nonsterile device and must be kept out of the sterile field (patient environment).
Device inspection. Before operating the system each time, inspect the clinician programmer and all its components for mechanical and electrical integrity. Avoid using the system if the clinician programmer or its components are damaged. If damage is observed, contact Technical Support.
Programmer use. Allow only authorized use of the clinician programmer to avoid any programming changes that may injure a patient.
Adverse Effects
In addition to those risks commonly associated with surgery, the following risks are associated with implanting or using this neurostimulation system:
- Unpleasant sensations or motor disturbances, including involuntary movement, caused by stimulation at high outputs (if either occurs, turn off stimulation immediately.)
- Undesirable changes in stimulation, which may be related to cellular changes in tissue around the electrodes, changes in electrode position, loose electrical connections, or lead failure
- Stimulation in unwanted places (such as radicular stimulation of the chest wall)
- Lead migration, causing changes in stimulation or reduced pain relief
- Epidural hemorrhage, hematoma, infection, spinal cord compression, or paralysis from placement of a lead in the epidural space
- Cerebrospinal fluid (CSF) leakage
- Paralysis, weakness, clumsiness, numbness, or pain below the level of the implant
- Persistent pain at the electrode or generator site
- Seroma (mass or swelling) at the generator site
- Allergic or rejection response to implant materials
- Implant migration or skin erosion around the implant
- Battery failure
- Changes in blood glucose levels in response to any adverse effect
NOTE: Patients with diabetes may have increased risks of infection, problems healing around the surgical site, and complications common to any surgical procedure. The severity of any surgical complication may be greater in patients with diabetes, particularly those with inadequate preoperative glycemic control. For adverse effects observed in the use of diabetic peripheral neuropathy, refer to the clinical summaries manual for SCS systems.
26-01562 MAT-2602037 v1.0 | Item approved for U.S. use only.
Prodigy™ and Proclaim™ SCS Systems
Prescription And Safety Information
Read this section to gather important prescription and safety information.
Intended Use
This neurostimulation system is designed to deliver low-intensity electrical impulses to nerve structures. The system is intended to be used with leads and associated extensions that are compatible with the system.
Indications For Use
This neurostimulation system is indicated as an aid in the management of chronic, intractable pain of the trunk and/or limbs, including unilateral or bilateral pain associated with the following: failed back surgery syndrome, intractable low back and leg pain, and diabetic peripheral neuropathy of the lower extremities.
Contraindications
This system is contraindicated for patients who are unable to operate the system or who have failed to receive effective pain relief during trial stimulation.
MRI Safety Information
Some models of this system are Magnetic Resonance (MR) Conditional, and patients with these devices may be scanned safely with magnetic resonance imaging (MRI) when the conditions for safe scanning are met. For more information about MR Conditional neurostimulation components and systems, including equipment settings, scanning procedures, and a complete listing of conditionally approved components, refer to the MRI procedures clinician's manual for neurostimulation systems (available online at medical.abbott/manuals).
For more information about MR Conditional products, visit the Abbott Medical product information page at neuromodulation.abbott/us/en/healthcare-professionals/mri-support.html.
Warnings
The following warnings apply to this neurostimulation system.
Poor surgical risks. Neurostimulation should not be used on patients who are poor surgical risks or patients with multiple illnesses or active general infections.
Magnetic resonance imaging (MRI). Some patients may be implanted with the components that make up a Magnetic Resonance (MR) Conditional system, which allows them to receive an MRI scan if all the requirements for the implanted components and for scanning are met. A physician can help determine if a patient is eligible to receive an MRI scan by following the requirements provided by Abbott Medical. Physicians should also discuss any risks of MRI with patients.
Patients without an MR Conditional neurostimulation system should not be subjected to MRI because the electromagnetic field generated by an MRI may damage the device electronics and induce voltage through the lead that could jolt or shock the patient.
Diathermy therapy. Do not use short-wave diathermy, microwave diathermy, or therapeutic ultrasound diathermy (all now referred to as diathermy) on patients implanted with a neurostimulation system. Energy from diathermy can be transferred through the implanted system and cause tissue damage at the location of the implanted electrodes, resulting in severe injury or death.
Diathermy is further prohibited because it may also damage the neurostimulation system components. This damage could result in loss of therapy, requiring additional surgery for system implantation and replacement. Injury or damage can occur during diathermy treatment whether the neurostimulation system is turned on or off.
Electrosurgery. To avoid harming the patient or damaging the neurostimulation system, do not use monopolar electrosurgery devices on patients with implanted neurostimulation systems. Before using an electrosurgery device, place the device in Surgery Mode using the patient controller app or clinician programmer app. Confirm the neurostimulation system is functioning correctly after the procedure.
During implant procedures, if electrosurgery devices must be used, take the following actions:
Use bipolar electrosurgery only.
Complete any electrosurgery procedures before connecting the leads or extensions to the neurostimulator.
Keep the current paths from the electrosurgery device as far from the neurostimulation system as possible.
Set the electrosurgery device to the lowest possible energy setting.
Confirm that the neurostimulation system is functioning correctly during the implant procedure and before closing the neurostimulator pocket.
Implanted cardiac systems. Physicians need to be aware of the risk and possible interaction between a neurostimulation system and an implanted cardiac system, such as a pacemaker or defibrillator. Electrical pulses from a neurostimulation system may interact with the sensing operation of an implanted cardiac system, causing the cardiac system to respond inappropriately. To minimize or prevent the implanted cardiac system from sensing the output of the neurostimulation system:
maximize the distance between the implanted systems;
Verify that the neurostimulation system is not interfering with the functions of the implanted cardiac system; and
Avoid programming either device in a unipolar mode (using the device’s can as an anode) or using neurostimulation system settings that interfere with the function of the implantable cardiac system.
Other active implanted devices. The neurostimulation system may interfere with the normal operation of another active implanted device, such as a pacemaker, defibrillator, or another type of neurostimulator. Conversely, the other active implanted device may interfere with the operation of the neurostimulation system.
Interference with other devices. Some of this system’s electronic equipment, such as the programmer and controller, can radiate radiofrequency (RF) energy that may interfere with other electronic devices, including other active implanted devices. Avoid placing equipment components directly over other electronic devices. To correct the effect of interference with other devices, turn off the equipment or increase the distance between the equipment and the device being affected.
Operation of machines, equipment, and vehicles. Patients using therapy that generates paresthesia should turn off stimulation before operating motorized vehicles, such as automobiles, or potentially dangerous machinery and equipment because sudden stimulation changes may distract them from properly operating it. However, current data shows that most patients using BurstDR™ stimulation therapy do not experience paresthesia. For patients who do not feel paresthesia, sudden stimulation changes are less likely to occur and distract them while operating motorized vehicles, machinery, or equipment.
Explosive and flammable gasses. Do not use a clinician programmer or patient controller in an environment where explosive or flammable gas fumes or vapors are present. The operation of these devices could cause them to ignite, causing severe burns, injury, or death.
Keep the device dry. Programmer and controller devices are not waterproof. Keep them dry to avoid damage. Advise patients to not use their device when engaging in activities that might cause it to get wet, such as swimming or bathing.
Pediatric use. Safety and effectiveness of neurostimulation for pediatric use have not been established.
Pregnancy and nursing. Safety and effectiveness of neurostimulation for use during pregnancy and nursing have not been established.
Use in patients with diabetes. Surgical complications and adverse events may be more frequent and severe in patients with diabetes. The following additional considerations should be made for patients with diabetes:
A pre-operative risk assessment should be performed for patients with diabetes who are at high risk for ischemic heart disease, those with autonomic neuropathy or renal failure, and patients with a Hemoglobin A1C (HbA1c) ≥8% (64 mmol/mol).
Monitor the patient’s blood glucose levels in the perioperative period and instruct the patient to continue to monitor glucose levels as they may fluctuate as a response to surgery or to complications. Implanting physicians or anesthesiologists should consult practice guidelines for the intraoperative management of patients with diabetes.
Closely monitor patients for signs of infection, delayed wound healing, or cerebrospinal fluid (CSF) leakage as the severity of these complications may be greater in patients with diabetes.
Stimulation Modes. The BurstDR™ stimulation mode has not been evaluated for effectiveness in the diabetic peripheral neuropathy (DPN) population.
Device components. The use of components not approved for use by Abbott Medical with this system may result in damage to the system and increased risk to the patient.
Device modification. Equipment is not serviceable by the customer. To prevent injury or damage to the system, do not modify the equipment. If needed, return the equipment to Abbott Medical for service
Application modification. To prevent unintended stimulation, do not modify the operating system in any way. Do not use the application if the operating system is compromised (that is, jailbroken).
Case damage. Do not handle the IPG if the case is pierced or ruptured because severe burns could result from exposure to battery chemicals.
IPG disposal. Return all explanted IPGs to Abbott Medical for safe disposal. IPGs contain batteries as well as other potentially hazardous materials. Do not crush, puncture, or burn the IPG because explosion or fire may result.
Product materials. Neurostimulation systems have materials that come in contact or may come in contact with tissue. A physician should determine whether or not a patient may have an allergic reaction to these materials before the system is implanted.
Precautions
The following precautions apply to this neurostimulation system.
General Precautions
Clinician training. Implanting physicians should be experienced in the diagnosis and treatment of chronic pain syndromes and have undergone surgical and device implantation training.
Patient selection. It is extremely important to select patients appropriately for neurostimulation. Thorough psychiatric screening should be performed. Patients should not be dependent on drugs and should be able to operate the neurostimulation system.
Infection. Follow proper infection control procedures. Infections related to system implantation might require that the device be explanted.
Implantation of two systems. If two systems are implanted, ensure that at least 20 cm (8 in) separates the implanted IPGs to minimize unintended interaction with other system components.
Implantation of multiple leads. If multiple leads are implanted, leads and extensions should be routed in close proximity. Nonadjacent leads can possibly create a conduit for stray electromagnetic energy that could cause the patient unwanted stimulation.
High stimulation outputs. Stimulation at high outputs may cause unpleasant sensations or motor disturbances, or render the patient incapable of controlling the stimulator. If unpleasant sensations occur, the device should be turned off immediately.
Electromagnetic interference (EMI). Some equipment in home, work, medical, and public environments can generate EMI that is strong enough to interfere with the operation of a neurostimulation system or damage system components. Patients should avoid getting too close to these types of EMI sources, which include the following examples: commercial electrical equipment (such as arc welders and induction furnaces), communication equipment (such as microwave transmitters and high-power amateur transmitters), high-voltage power lines, radiofrequency identification (RFID) devices, and some medical procedures (such as therapeutic radiation and electromagnetic lithotripsy).
Lead movement. Patients should be instructed to avoid bending, twisting, stretching, and lifting objects over 2 kg (5 lb) six to eight weeks after implantation of a neurostimulation system. Extension of the upper torso or neck may cause lead movement and alter the stimulation field (especially with leads in the cervical area), resulting in overstimulation or ineffective stimulation.
Patient training. Instruct patients to use their neurostimulation system only after an authorized clinician has programmed the device and has trained the patient how to control stimulation and safely use the system.
Programmer use. Allow only authorized use of the clinician programmer to avoid any programming changes that may injure a patient.
Sterilization and Storage
Single-use, sterile device. The implanted components of this neurostimulation system are intended for a single use only. Sterile components in this kit have been sterilized using ethylene oxide (EtO) gas before shipment and are supplied in sterile packaging to permit direct introduction into the sterile field. Do not resterilize or reimplant an explanted system for any reason.
Storage environment. Store components and their packaging where they will not come in contact with liquids of any kind.
Handling and Implementation
Expiration date. An expiration date (or “use-before” date) is printed on the packaging. Do not use the system if the use-before date has expired.
Handle the device with care. The clinician programmer and patient controller are sensitive electronic devices that can be damaged by rough handling, such as dropping them on the ground.
Care and handling of components. Use extreme care when handling system components prior to implantation. Excessive heat, excessive traction, excessive bending, excessive twisting, or the use of sharp instruments may damage and cause failure of the components.
Package or component damage. Do not implant a device if the sterile package or components show signs of damage, if the sterile seal is ruptured, or if contamination is suspected for any reason. Return any suspect components to Abbott Medical for evaluation.
Exposure to body fluids or saline. Prior to connection, exposure of the metal contacts, such as those on the connection end of a lead or extension, to body fluids or saline can lead to corrosion. If such exposure occurs, clean the affected parts with sterile, deionized water or sterile water for irrigation, and dry them completely prior to lead connection and implantation.
System testing. To ensure correct operation, always test the system during the implant procedure, before closing the neurostimulator pocket, and before the patient leaves the surgery suite.
Hospitals and Medical Environments
High-output ultrasonics and lithotripsy. The use of high-output devices, such as an electrohydraulic lithotripter, may cause damage to the electronic circuitry of an implanted IPG. If lithotripsy must be used, do not focus the energy near the IPG.
Ultrasonic scanning equipment. The use of ultrasonic scanning equipment may cause mechanical damage to an implanted neurostimulation system if used directly over the implanted system.
External defibrillators. The safety of discharge of an external defibrillator on patients with implanted neurostimulation systems has not been established.
Therapeutic radiation. Therapeutic radiation may damage the electronic circuitry of an implanted neurostimulation system, although no testing has been done and no definite information on radiation effects is available. Sources of therapeutic radiation include therapeutic X rays, cobalt machines, and linear accelerators. If radiation therapy is required, the area over the implanted IPG should be shielded with lead. Damage to the system may not be immediately detectable.
Home and Occupational Environments
Security, antitheft, and radiofrequency identification (RFID) devices. Some antitheft devices, such as those used at entrances or exits of department stores, libraries, and other public places, and airport security screening devices may affect stimulation. Additionally, RFID devices, which are often used to read identification badges, as well as some tag deactivation devices, such as those used at payment counters at stores and loan desks at libraries, may also affect stimulation. Patients who are implanted with nonadjacent multiple leads and patients who are sensitive to low stimulation thresholds may experience a momentary increase in their perceived stimulation, which some patients have described as uncomfortable or jolting.
Patients should cautiously approach such devices and should request help to bypass them. If they must go through a gate or doorway containing this type of device, patients should turn off their IPG and proceed with caution, being sure to move through the device quickly.
Scuba diving or hyperbaric chambers. Patients should not dive below 30 m (100 ft) of water or enter hyperbaric chambers above 4.0 atmospheres absolute (ATA). Pressures below 30 m (100 ft) of water (or above 4.0 ATA) could damage the neurostimulation system. Before diving or using a hyperbaric chamber, patients should discuss the effects of high pressure with their physician.
Wireless use restrictions. In some environments, the use of wireless functions (e.g., Bluetooth® wireless technology) may be restricted. Such restrictions may apply aboard airplanes, in hospitals, near explosives, or in hazardous locations. If you are unsure of the policy that applies to the use of this device, please ask for authorization to use it before turning it on. (Bluetooth® is a registered trademark of Bluetooth SIG, Inc.)
Consumer goods and electronic devices. Magnetic interference with consumer goods or electronic devices that contain magnets, such as mobile phones and smart watches, may unintentionally cause the neurostimulation system to turn on or turn off or affect communication between the device and generator; however, it will not change the prescribed programmed parameters.
Patients should be advised to keep their mobile phones and smart watches at least 15 cm (6 in.) away from the generator and avoid placing any smart device in a pocket near the generator. If a patient is concerned about a smart device interacting with their neurostimulation system, consider disabling magnet mode. For more information about setting the magnet mode, refer to the clinician programmer manual or contact Technical Support.
Mobile phones. While interference with mobile phones is not anticipated, technology continues to change and interaction between a neurostimulation system and a mobile phone is possible. Advise patients to contact their physician if they are concerned about their mobile phone interacting with their neurostimulation system.
Adverse Effects
In addition to those risks commonly associated with surgery, the following risks are associated with implanting or using this neurostimulation system:
Unpleasant sensations or motor disturbances, including involuntary movement, caused by stimulation at high outputs (If either occurs, turn off your IPG immediately.)
Undesirable changes in stimulation, which may be related to cellular changes in tissue around the electrodes, changes in electrode position, loose electrical connections, or lead failure
Stimulation in unwanted places (such as radicular stimulation of the chest wall)
Lead migration, causing changes in stimulation or reduced pain relief
Epidural hemorrhage, hematoma, infection, spinal cord compression, or paralysis from placement of a lead in the epidural space
Cerebrospinal fluid (CSF) leakage
Paralysis, weakness, clumsiness, numbness, or pain below the level of the implant
Persistent pain at the electrode or IPG site
Seroma (mass or swelling) at the IPG site
Allergic or rejection response to implant materials
Implant migration or skin erosion around the implant
Battery failure
Changes in blood glucose levels in response to any adverse effect
Note: Patients with diabetes may have increased risks of infection, problems healing around the surgical site, and complications common to any surgical procedure. The severity of any surgical complication may be greater in patients with diabetes, particularly those with inadequate pre-operative glycemic control. For adverse effects observed in SCS clinical studies, refer to the clinical summaries manual for SCS systems.
Safety And Effectiveness Studies
For information that supports the clinical use of this neurostimulation system, refer to the clinical summaries manual for spinal cord stimulation (SCS) systems (available online at medical.abbott/manuals). This neurostimulation system is similar in technology and intended use to the systems reported in the literature and clinical studies. Therefore, the literature and clinical studies represent the safety and effectiveness of this neurostimulation system.
21 CR 801.109(b) The label of the device, other than surgical instruments, bears:
(1) The symbol statement “Rx only” or “℞ only” or the statement “Caution: Federal law restricts this device to sale by or on the order of a ___”, the blank to be filled with the word “physician,” “dentist,” “veterinarian,” or with the descriptive designation of any other practitioner licensed by the law of the State in which the practitioner practices to use or order the use of the device; and
(2) The method of its application or use.
74373 MAT-2300644 v2.0 | Item approved for U.S. use only.
25-111814 MAT-2305143 v6.0 | Item approved for U.S. use only.



Chronic Pain